
Identify the breast cancer risk of all women worldwide
Breast cancer, the 1st cancer in the world among women
2,3 millions
670 000
Our goal:
reduce mortality by 20%**
MammoRisk® innovation
Currently, at-risk women are not identified. Additionally,70 to 80% of breast cancers occur inwomen without a family history1.
MammoRisk® is a Class I medical device that evaluates the risk of invasive breast cancer based on 5 main risk factors: age, family history, biopsy history, polygenic score2, and breast density.
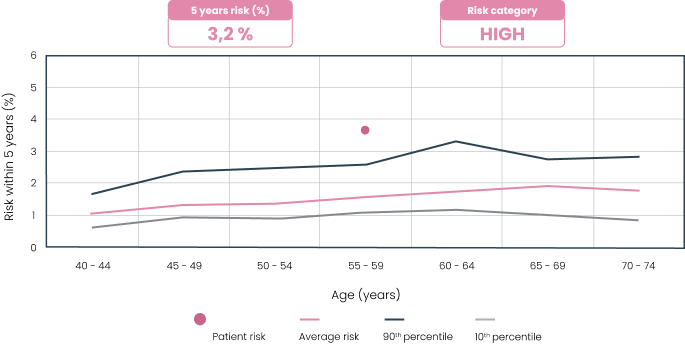
The MammoRisk® test provides a clear and precise report that estimates the absolute risk of breast cancer over 5 years. Based on this “risk score,” the doctor will establish a personalized follow-up program in shared decision-making with the patient (screening frequency and types, Hormone Replacement Therapies, etc.).

- Mammogram start age: 40 or 50 years
- Mammogram frequency : once a year, once every 2 years, or none before age 50
- Annual MRI for a lifetime risk above 20% in the United States, approximately 10% of eligible women3
How does it work?
MammoRisk®, is the first breast cancer risk prediction test based on3 pillars and a patented AI method4.
Genetic test with saliva sample
on medical prescription
Medical imaging
Patient’s clinical data
Neighbor method4
Artificial Intelligence
MammoRisk® a high-performing and readable model.
Our model is based on an algorithm that identifies “digital twins” for each patient: age, personal and family history, breast density, to calculate an ultra-personalized risk of disease occurrence.
See the press release on the patent
2
3 Sur prescription médicale
4 Méthode brevetée pouvant s’adapter à toutes les bases de données en intégrant des paramètres cliniques, d’imagerie ou de génétique.
Mammorisk® an international recognition
Mammorisk® selected by the European Unione 
Our test has been exclusively selected for the only randomized clinical trial in the worldon breast cancer screening for women over 40: the MyPeBS* study (My Personal Breast Screening).
This study aims to evaluate whether personalized breast cancer screening could be a better option for women aged 40 to 70 and aims to eventually change the screening of more than 100 million European women.
Learn more about the MyPeBS study

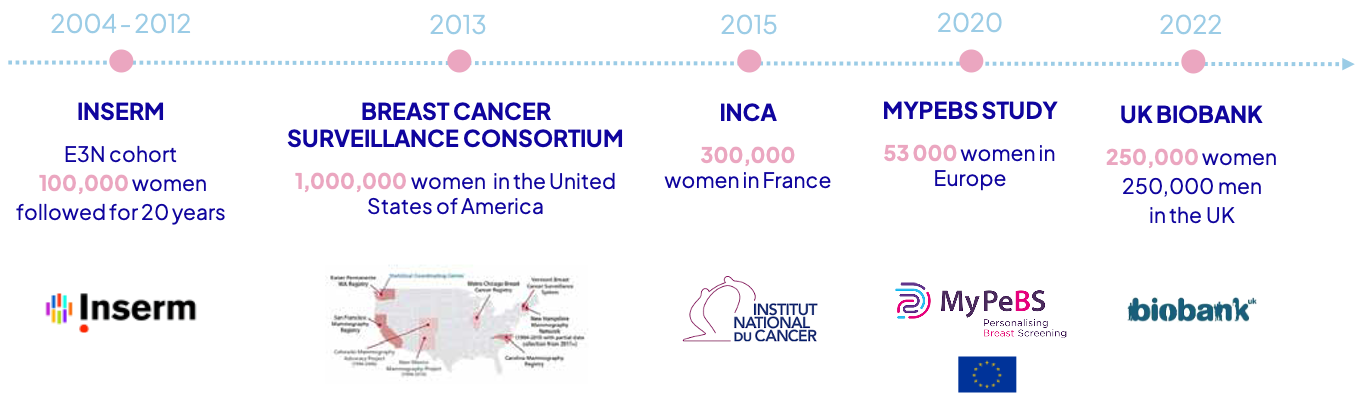
A strong entry barrier: access to databases of over 2 million people
The first breast cancer prediction model was developed from 100,000 French women using a simple and visual method* now patented, consisting of observing the outcomes of a woman’s “behavioral neighbors.” This model, named Mammorisk® with early AI elements, was then exported in 2013 to a database of 1,000,000 American women, allowing us to validate its effectiveness more broadly. This Franco-American model was then successfully tested on 300,000 French women grouped by the initiative of the Gustave Roussy Institute, validating its applicability worldwide.
The market
MammoRisk®,a Blockbuster in coming.
Breast cancer risk prediction is aimed at all women over the age of 40, in France and throughout the world. Unlike treatment, prevention is aimed at a much wider population, encompassing all healthy women. This predictive test therefore has the potential to generate a market of considerable scope, given its scale and positive impact on public health.
200 millions concerned women
About us

« When I was teaching applied mathematics to medicine and working at the Gustave Roussy Cancer Center, I met patients in the hallways and wondered if we could have anticipated these pathologies. That’s where the idea of predicting risks to better prevent them was born »
Stéphane Ragusa
CEO
Former student of École Polytechnique
Mammorisk is the fruit of 20 years of R&D. Founded in 2004, Predilife, a pioneer in predictive medicine in France, designs and markets predictive tests, enabling each individual to define his or her risk profile with regard to the occurrence of serious illnesses.
It combines proven medical techniques with Artificial Intelligence and Big Data.
Predictive medicine consists in anticipating an individual’s future so as to act in good time thanks to personalized prevention, either by reducing risks or identifying illness earlier.
Serious pathologies, such as cancer and heart attacks, can now be better prevented.
The Management Team

Stéphane RAGUSA
PRESIDENT-FOUNDER I CEO

Anne KERZAN
CHIEF MARKETING OFFICER

Émilien GAUTHIER
CHIEF TECHNICAL OFFICER

Marion RENELIER
LEGAL DIRECTOR I DATA PROTECTION OFFICER
Our Partners



Our Approvals and Certifications
Our medical device is registered with ANSM.
Our medical device complies with essential requirements set out in European regulations (CE marking).

Ce qu’en pensent les femmes
- "I greatly appreciated this assessment, and the medical support was of good quality. I felt supported, which allowed me to initiate steps I wouldn’t have dared to take alone."
- "Smooth, clear, and efficient process. Reassuring assessment that gave me peace of mind."
- "Simple, quick, and so useful"
